How to Use: FODZYME Clinical Support Tool
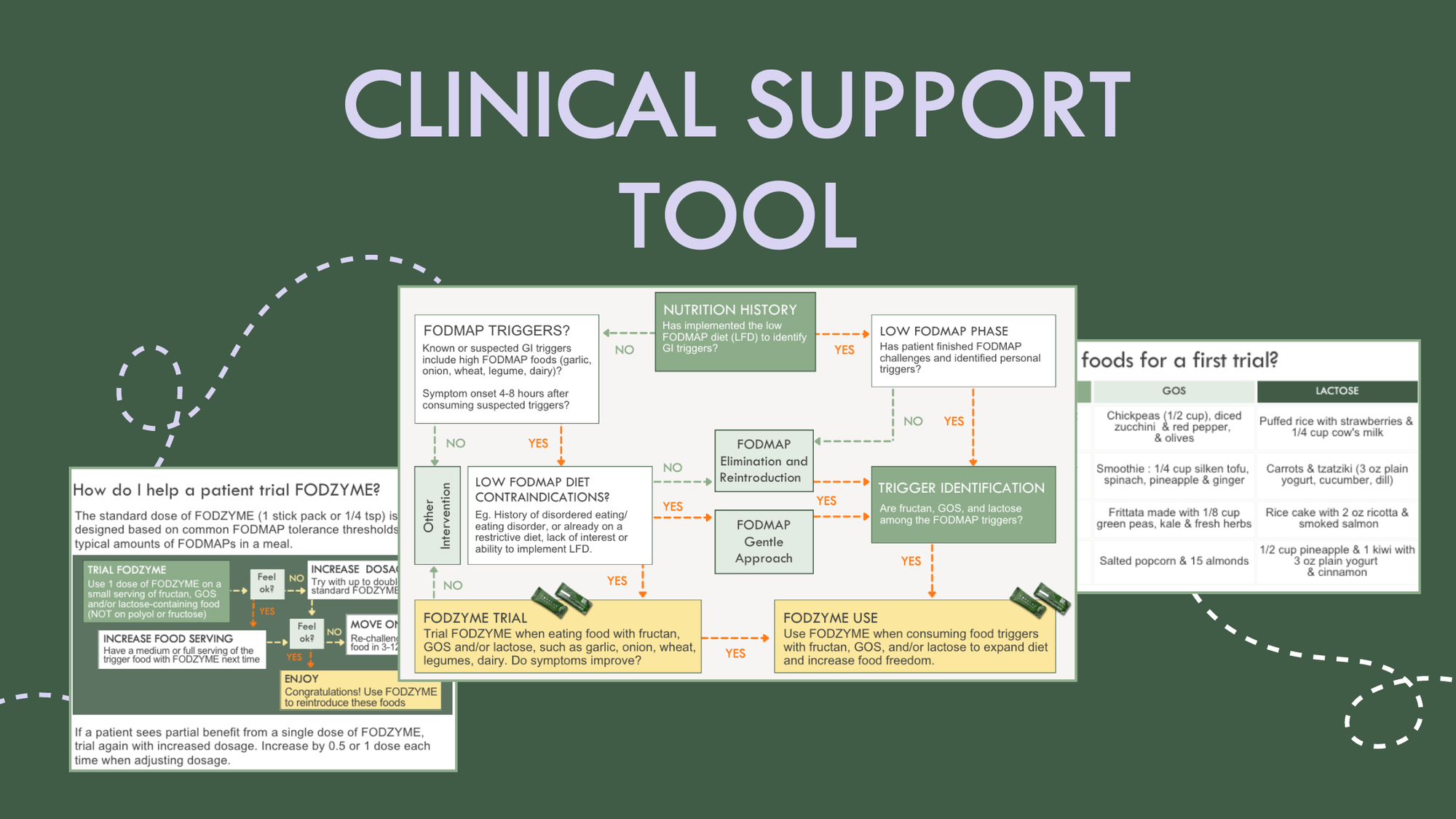
The FODZYME Clinical Support Tool, co-created with Jessie Wong, MAcc, RDN, LD, provides a structured framework to integrate FODZYME into your care process.
FODZYME's digestive enzymes are a highly versatile way to support dietary freedom and symptom control for FODMAP sensitive patients.
FODZYME can be used a) prior to the full low FODMAP diet (LFD), b) following a FODMAP 'Gentle' approach or c) during the third phase of a formal LFD. There's no ‘right’ or ‘wrong’ way to use FODZYME, but assessing which approach is appropriate for each patient takes nuance and clinical judgement.
The FODZYME Clinical Support Tool, co-created with Jessie Wong, MAcc, RDN, LD, provides a structured framework to integrate FODZYME into your care process.
FODZYME can be a useful option for a wide range of patients. Our aim is to help you feel confident applying your clinical judgement to determine when and how to use digestive enzymes in your practice. In this blog, we'll review common patient presentations and when and how to use FODZYME with each.
DOWNLOAD CLINICAL SUPPORT TOOL
Orientation: Diagnostic Algorithms
Clinical support tools, sometimes referred to as diagnostic algorithms, ensure delivery of the most appropriate patient care. They are used to guide assessments and interventions and are especially useful in patients where there are many potential management strategies to consider.
This is often the case in those with bloating, gas, constipation, diarrhea and other digestive symptoms. These patients are often also in the early stages of understanding the extent to which FODMAPs may play a role in their symptoms. For some, the full LFD will be appropriate to help patients fully understand their triggers and tolerance levels, while for others the simple mention of garlic, onion and beans will be enough to identify their biggest FODMAP triggers and guide your first-line intervention.
The FODZYME Clinical Support Tool provides a framework to identify appropriate candidates for FODZYME depending on their clinical indications and a step-by-step guide on how to introduce FODZYME into their diet.
Patient 1: Contraindication for the low FODMAP Diet
Those with a history of an eating disorder or at risk for disordered eating are contraindicated for the LFD. This first patient scenario explains why and illustrates how to use the Clinical Support Tool in this population.

Among patients with gastrointestinal (GI) conditions, the risk for disordered eating and food fears and anxiety is unfortunately high. Restrictive diets, such as the LFD, should be applied with careful consideration only after risk for disordered eating has been deemed low [1].
Screening for Disordered Eating
Registered Dietitians play a key role in screening patients to identify disordered eating behaviors. Following assessment of risk for disordered eating, selection of appropriate interventions for patients may or may not include dietary modification, depending on a patient's responses during the screen. Screening for disordered eating behaviors also guides potential referral to other interdisciplinary team members and providers.
While there are few screening tools validated to screen for disordered eating behaviors within the GI population, the Fear of Food Questionnaire (FFQ-18) and the Food-Related Quality of Life (FRQOL) are available.
Due to the lack of validated tools for screening, clinical judgement is important during intake and assessment. Open-ended questions help reveal a patient's relationship to food and the potential impact of a restrictive diet. Areas to probe when assessing risk for disordered eating may include:
Describe your relationship with food
Assess for:
- Feelings of anxiety around food
- Fear of adding foods to the diet
- Extent of food avoidance to manage digestive symptoms
- Reluctance or unwillingness to introduce additional foods and assess tolerance
Tell me about your behaviors around food
Assess for:
- Avoidance of social situations that involve food or that may include lack of control over food
- Infrequent or skipped meals; inadequate intake overall
- Extensive amount of time spent thinking about food and its impact on daily life
Describe how you think an elimination diet may impact you
Assess response for suggestion that it may:
- Increase obsessiveness about food and food rules
- Impose excessive limitations on social and daily life
- Provoke moral associations with food (eg. 'good/bad' 'safe/unsafe')
Those without a formal diagnosis, as well as those who show a lack of willingness or capability to understand or apply the LFD are also contraindicated. Still, these patients may benefit from trialing FODZYME if they describe a diet high in FODMAPs and their symptom descriptions suggest FODMAPs may play a role.
FODZYME Integration
Application of the the Clinical Support Tool in a population contraindicated for a LFD often follows these steps:
- Assessment of nutrition history to learn that a patient has not trialed a LFD to date.
- Discussion of triggers and development of hypothesis that FODMAPs may be triggering through conversations on common foods consumed, their known or suspected impact on GI symptoms and timing of symptom onset.
- Screen for disordered eating risk. If patients appears high risk, a LFD is contraindicated.
- Trial of FODZYME with foods high in fructan, GOS and/or lactose that are known or suspected to trigger symptoms (note that a FODMAP 'Gentle' approach could be used here; see below). Then, assess response for symptom improvement (remember: FODMAPs typically trigger symptoms when they reach the colon, 4-8 hours after a meal).
Patient 2: FODMAP 'Gentle' Approach
Patients who are contraindicated for the LFD or uninterested in it may be suitable for a FODMAP 'Gentle' approach. This next patient scenario illustrates the benefits of a FODMAP 'Gentle' approach and how to use FODZYME with candidates for this method.
About FODMAP 'Gentle'
The traditional LFD uses a 'top-down' approach to identify triggers. This includes FODMAP restriction for 2-6 weeks to assess for symptom benefit, followed by systematic food 'challenges' and then diet personalization.
In contrast, a FODMAP 'Gentle' approach uses a 'bottom-up' strategy to reduce a select set of foods that are very high in FODMAPs based on a patient's diet [2]. Similar to the traditional LFD, this first phase is limited in duration only as long as needed to understand if there is a symptom benefit.
Common foods limited in the FODMAP 'Gentle' approach include [1]:
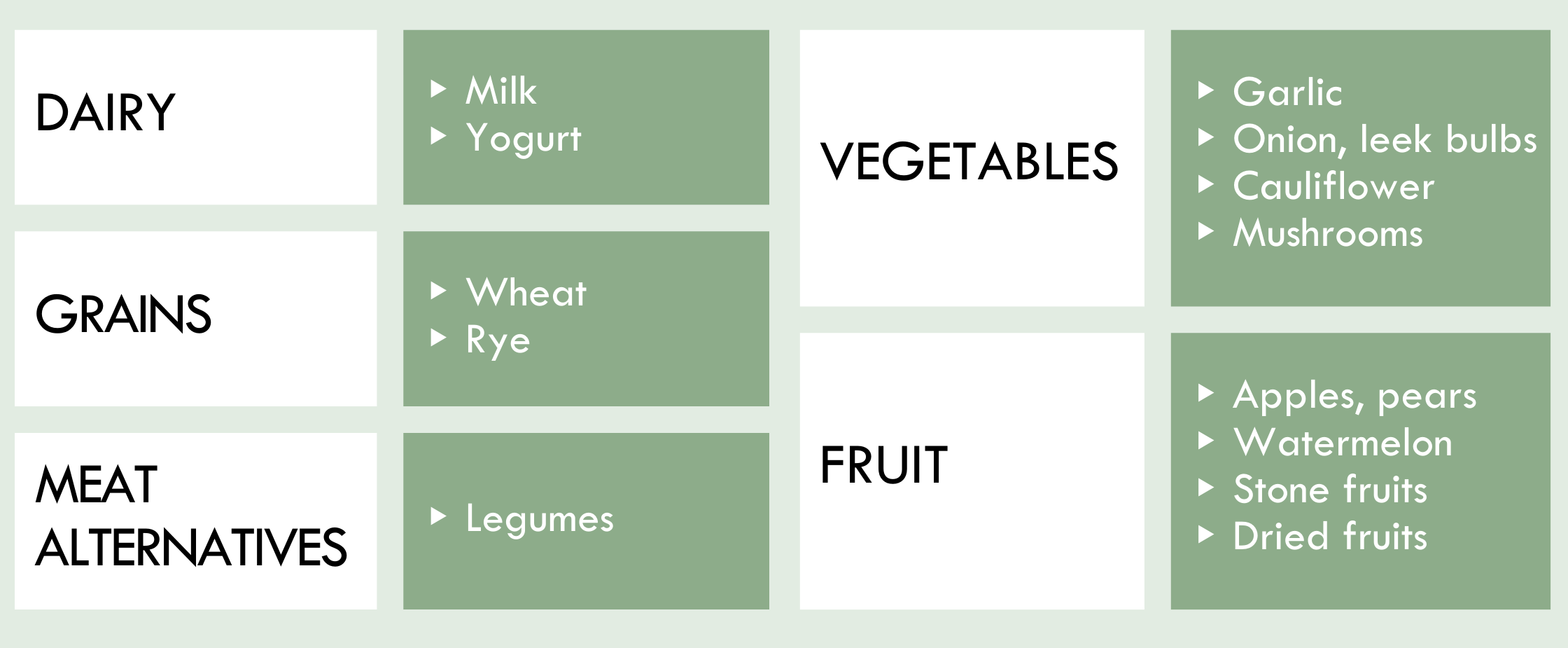
If there is symptom improvement following reduction of these foods, a patient would move to phases two and three of the FODMAP 'Gentle' approach to learn personal triggers through food 'challenges' and then ultimately develop a long-term, personalized diet.
A variety of patients benefit from the FODMAP 'Gentle' approach. This includes those with or at risk for eating disorders, patients with malnutrition or other pre-existing dietary restrictions, children, patients with comorbidities that pose negative risk associated with diet restrictions (e.g. IBD and pregnancy) and/or those who show a lack of willingness or capability to apply or understand the LFD [1].
Increasingly, a FODMAP 'Gentle' approach is used in clinical practice for a wide variety of patients beyond those with a formal contraindication.
Significant symptom relief can often be achieved with targeted FODMAP elimination, as a small group of FODMAP subgroups generally represent the majority of FODMAP intolerances and may be responsible for most symptoms (eg. fructans in garlic, onion and wheat, mannitol in cauliflower and mushrooms and GOS/galactans in legumes) [3].
FODZYME Integration
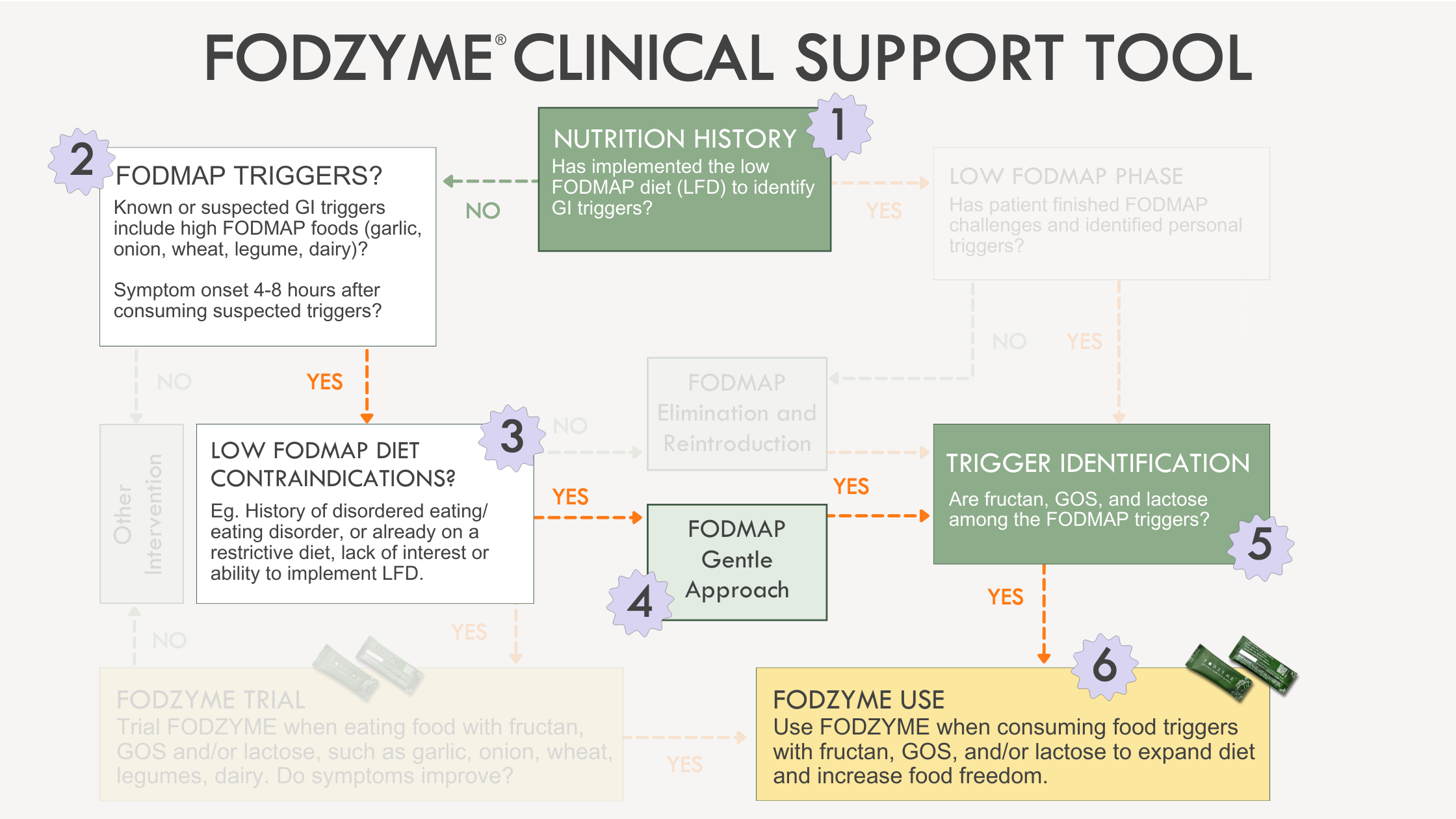
Application of the the Clinical Support Tool in a population who can benefit from a FODMAP 'Gentle' approach follows these steps:
- Nutrition history noting the patient has not trialed a LFD.
- Discussion of triggers and development of hypothesis that FODMAPs may be triggering based on typical diet and relationship between food intake and symptom onset, noting high amount of FODMAPs in the typical diet and digestive symptoms 4-8 hours after meals.
- Screening and identification of risk for disordered eating.
- Trial of 'FODMAP' gentle by eliminating garlic, onion, wheat, legumes, high fructose fruits, cauliflower, mushrooms and lactose-containing dairy. Agreement with patient to monitor symptoms for 2-6 weeks and assess symptom response.
- Notable improvement in symptoms, warranting progression to 'challenge' each FODMAP subgroup to identify specific FODMAP triggers and portions tolerated (note: reference the Monash App and Monash Guidelines for more resources to support patients through Reintroduction)
- Identification of fructan, GOS, and/or lactose as triggers, warranting use of FODZYME during personalization phase to expand diet and increase food freedom.
Patient 3: The Traditional Low FODMAP Diet
While the LFD is a three-phased diet, far too many patients unfortunately never make it past phase one and do not move on to reintroduction and diet personalization. Consequences of staying in phase one of the LFD for too long pose serious nutritional risks and consequences for quality of life.
This next scenario reviews the importance of implementing all three phases of the LFD and how to guide patients through this approach when integrating FODZYME.
Personalization Phase
Risks of excessive dietary restriction and failure to move past phase one of the LFD include nutrient and energy deficiencies, increased food anxiety and fears, higher food costs, social isolation and disruptions to gut microbiota.
Excessive dietary restriction increases risk for nutrient and energy deficiencies, food anxiety and fears and social isolation.
After achieving symptom relief in phase one of the LFD, patients may become reluctant to move on to phases two and three of the diet due to lack of knowledge on proper implementation of the LFD and/or fear that symptoms will return. However, it's vital that patients move on to phase two to learn their unique triggers and tolerances and develop a personalized diet that is sustainable, nutritionally adequate and supportive of mental wellbeing.
Once a patient reaches phase three of the LFD, personalization, FODZYME can be used with trigger foods high in fructan, GOS and/or lactose. A large body of research supports the use of digestive enzymes as a safe and effective tool to manage digestive symptoms like gas, bloating and more [4-8].
FODZYME Integration
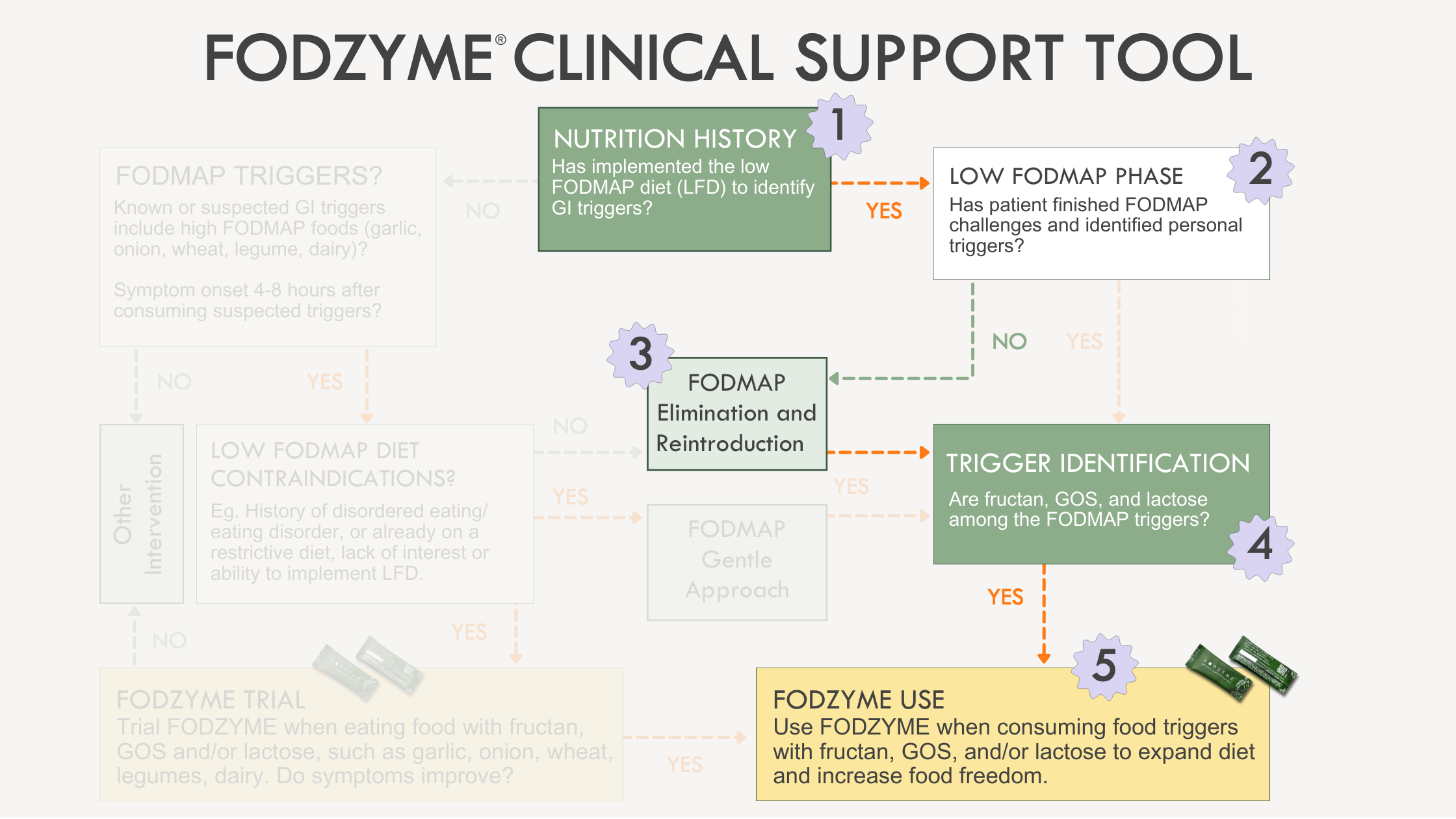
Application of the the Clinical Support Tool in a population following a traditional LFD approach follows these steps:
- Nutrition history noting the patient is a suitable candidate for the LFD.
- Patient education discussing the three phases of the diet and the purpose of each.
- Implementation of phases two and three of the LFD: FODMAP Elimination and Reintroduction.
- Identification of fructan, GOS, and lactose among FODMAP triggers.
- Diet personalization using FODZYME to allow for liberal consumption of the FODMAP triggers fructan, GOS, and/or lactose based on diet preferences and lifestyle.
Conclusion
Identification of FODMAP triggers is a complex process. It requires close collaboration and clinical judgement to identify the best approach for each patient.
Remember: FODMAP intolerances exist along a spectrum and may or may not be associated with a formal diagnosis.
A diagnostic algorithm, like the FODZYME Clinical Support Tool, will guide your application of the various methods to identify FODMAP triggers.
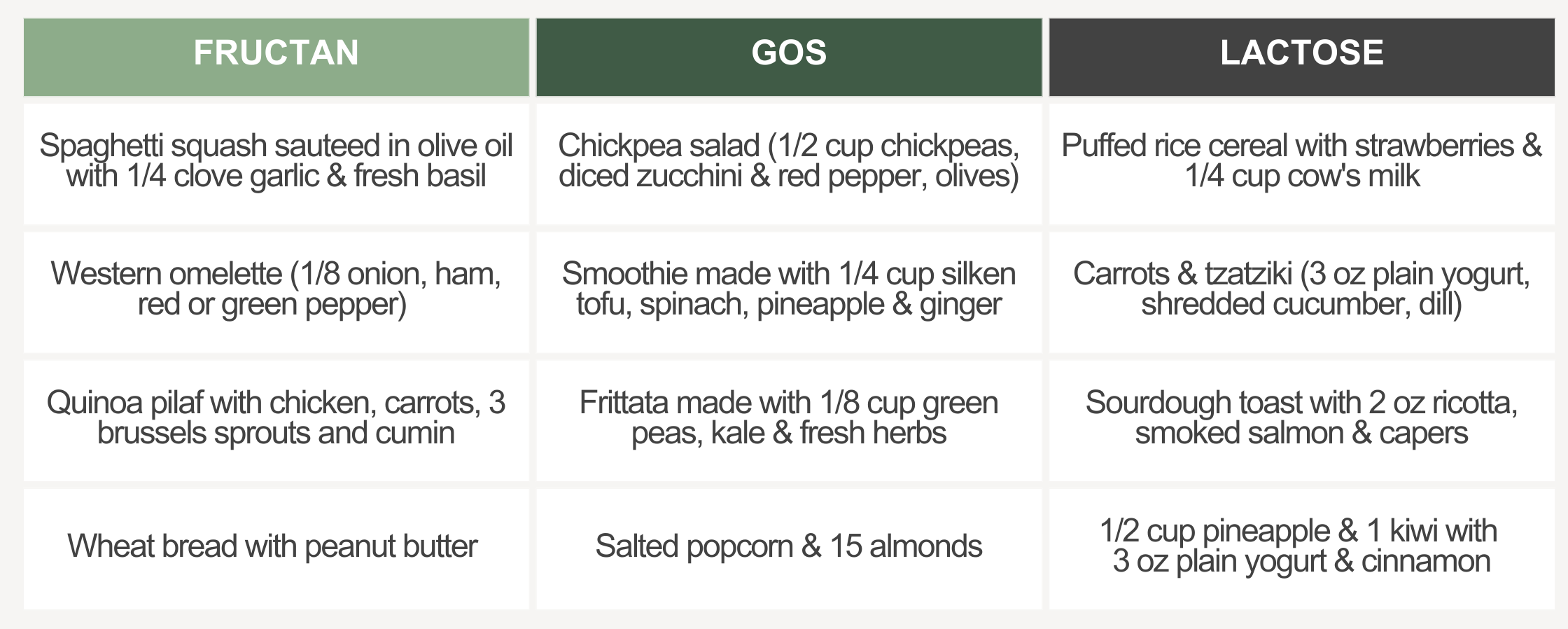
For those who suspect or learn FODMAPs are triggering, FODZYME can support dietary freedom. The tool includes a step-by-step guide and suggested meals and snacks for their first trial.
Meal suggestions for a first FODZYME trial
DOWNLOAD CLINICAL SUPPORT TOOL
Continue your Learning
In this recording from our live Lunch & Learn, Jessie shares more guidance on how to apply the Clinical Support Tool and presents patient cases where FODZYME facilitated dietary expansion and symptom control:
About Jessie Wong, MAcc, RDN, LD
Jessie is a Monash trained RDN with years of experience helping people find relief and freedom from IBS. She is the creator of the IBS Freedom Approach, where her mission is to ensure nobody has to let their symptoms dictate their life. The IBS Freedom Approach is easy-to-follow, proven for IBS relief, and accessible anywhere around the world.
References:
- Halmos EP, Gibson PR. Controversies and reality of the FODMAP diet for patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2019;34(7):1134-1142. doi:10.1111/jgh.14650
- Halmos EP. When the low FODMAP diet does not work. J Gastroenterol Hepatol. 2017;32 Suppl 1:69-72. doi:10.1111/jgh.13701
- Van den Houte K, Colomier E, Routhiaux K, et al. Efficacy and Findings of a Blinded Randomized Reintroduction Phase for the Low FODMAP Diet in Irritable Bowel Syndrome. Gastroenterology. Published online February 23, 2024. doi:10.1053/j.gastro.2024.02.008
- Tuck CJ, Taylor KM, Gibson PR, Barrett JS, Muir JG. Increasing Symptoms in Irritable Bowel Symptoms With Ingestion of Galacto-Oligosaccharides Are Mitigated by α-Galactosidase Treatment. American Journal of Gastroenterology. 2018;113(1):124-134. doi:https://doi.org/10.1038/ajg.2017.245
- Di Stefano M, Miceli E, Gotti S, Missanelli A, Mazzocchi S, Corazza GR. The Effect of Oral α-Galactosidase on Intestinal Gas Production and Gas-Related Symptoms. Digestive Diseases and Sciences. 2006;52(1):78-83. doi:https://doi.org/10.1007/s10620-006-9296-9
- Di Nardo G, Oliva S, Ferrari F, et al. Efficacy and tolerability of α-galactosidase in treating gas-related symptoms in children: a randomized, double-blind, placebo controlled trial. BMC Gastroenterology. 2013;13(1). doi:https://doi.org/10.1186/1471-230x-13-142
- Lin MY, Dipalma JA, Martini MC, Gross CJ, Harlander SK, Savaiano DA. Comparative effects of exogenous lactase (?-galactosidase) preparations on in vivo lactose digestion. Digestive Diseases and Sciences. 1993;38(11):2022-2027. doi:https://doi.org/10.1007/bf01297079
- Ibba I, Gilli A, Boi MF, Usai P. Effects of Exogenous Lactase Administration on Hydrogen Breath Excretion and Intestinal Symptoms in Patients Presenting Lactose Malabsorption and Intolerance. BioMed Research International. 2014;2014:1-7. doi:https://doi.org/10.1155/2014/680196

